Synthesis of Functional Quinoxaline Derivatives for the Application in Biology and Materials Sciences
Seiten
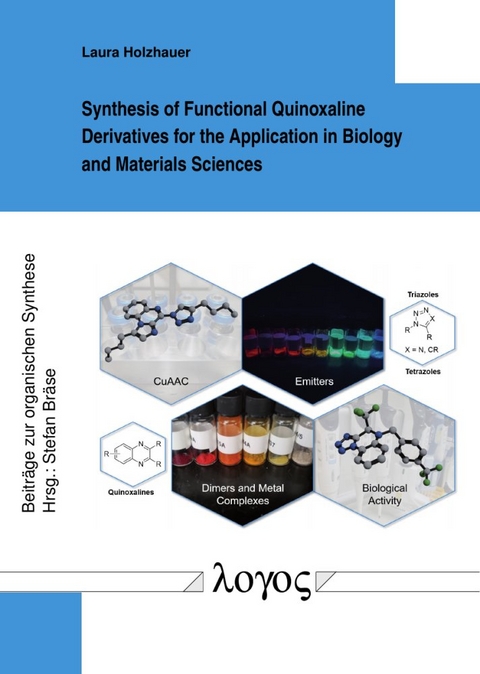
Nitrogen-containing heterocycles belong to the most versatile and important compounds for medicinal chemistry and sustainable and advanced materials. Around 60 % of FDA-approved unique small molecule drugs incorporate a nitrogen heterocycle. N-heterocyclic compounds are essential components of organic light-emitting diodes and organic solar cells. Quinoxalines are a class of heterocyclic compounds accessible by straightforward syntheses, present in commercially available drugs and optoelectronic architectures.
In this work, quinoxaline-containing compounds were synthesized and analyzed regarding their potential uses in biology and materials science, focusing on nitrogen-rich and dimeric quinoxalines. Various tetrazolo[1,5-a]quinoxalines were synthesized and converted to 1,2,3-triazoloquinoxalines and novel triazoloimidazoquinoxalines via a modified CuAAC procedure. A previously unknown copper-catalyzed denitrogenative annulation process was described in this context. As tankyrase inhibitors, substituted tetrazolo[1,5-a]quinoxaline derivatives were assembled and tested in biological studies. Moreover, the synthesis of quinoxaline dimers and metal complexation of selected 1,2,3-triazoloquinoxalines was investigated. Donor-acceptor emitter structures were assembled employing suitable tetrazolo [1,5-a]quinoxalines and 1,2,3-triazoloquinoxalines and their emissive properties were explored, highlighting the influence of the direction of the triazole linker on delayed fluorescence.
In this work, quinoxaline-containing compounds were synthesized and analyzed regarding their potential uses in biology and materials science, focusing on nitrogen-rich and dimeric quinoxalines. Various tetrazolo[1,5-a]quinoxalines were synthesized and converted to 1,2,3-triazoloquinoxalines and novel triazoloimidazoquinoxalines via a modified CuAAC procedure. A previously unknown copper-catalyzed denitrogenative annulation process was described in this context. As tankyrase inhibitors, substituted tetrazolo[1,5-a]quinoxaline derivatives were assembled and tested in biological studies. Moreover, the synthesis of quinoxaline dimers and metal complexation of selected 1,2,3-triazoloquinoxalines was investigated. Donor-acceptor emitter structures were assembled employing suitable tetrazolo [1,5-a]quinoxalines and 1,2,3-triazoloquinoxalines and their emissive properties were explored, highlighting the influence of the direction of the triazole linker on delayed fluorescence.
| Erscheinungsdatum | 16.03.2024 |
|---|---|
| Reihe/Serie | Beiträge zur organischen Synthese ; 105 |
| Sprache | englisch |
| Maße | 145 x 210 mm |
| Einbandart | Paperback |
| Themenwelt | Naturwissenschaften ► Chemie ► Organische Chemie |
| Schlagworte | Azides • Heterocycles • Luminescence • Organic Chemistry • Tetrazoles and Triazoles |
| ISBN-13 | 9783832557799 / 9783832557799 |
| Zustand | Neuware |
| Informationen gemäß Produktsicherheitsverordnung (GPSR) | |
| Haben Sie eine Frage zum Produkt? |
Mehr entdecken
aus dem Bereich
aus dem Bereich